Clean Rooms
Objetivo Marte is an Engineering & Trading Company
Guidelines
Objetivo Marte has a Partnership with a Spanish Company.
We design and install customized cleanrooms. A clean room is understood to be a closed space that allows extremely low levels of contamination to be maintained. They are used in practically every industry where small particles can adversely affect the manufacturing process.
Clean rooms are designed to reduce contamination as much as possible. Clean rooms are also be considered those that, despite not being so demanding with the purity of the air present very strict requirements regarding the absence of electromagnetic fields.
White rooms can carry out production without running the risk of cross contamination from any type of pathogenic agents. The presence of microorganisms in the environment can alter the properties of certain products. For example, in the case of food (whether meat, fish, dairy, vegetables or precooked), it can pose a risk to people’s health, causing poisoning and infections.
They are very common in the development of products in Pharmaceutical Industry, although they are also used in areas such as: Cannabis, Hospitals, Veterinary, Food Industry, Cosmetics, Biological Safety Cabinets.

Regulation Standards
It’s essential that both the design and the construction of the room white is carried out by professionals in this field. Otherwise, it will be very difficult to obtain authorization to manufacture regulated products under the regulations.
ISO STANDART
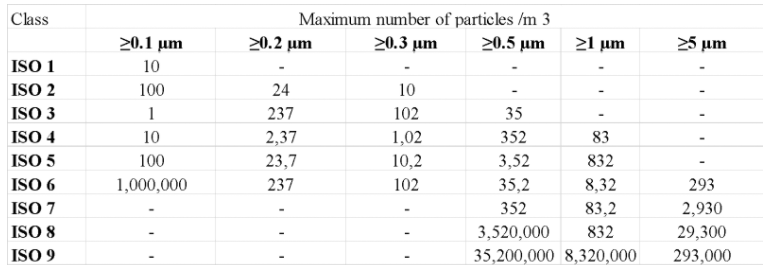
ISO 14644-1 (International Standards Organization) classifies cleanrooms, according to the number of particles in the air.
ISO 14644-1: Classification of air cleanliness.
ISO 14644-2: Specifications for tests.
ISO 14644-3: Test methods.
ISO 14644-4: Design, construction, and commissioning.
ISO 14644-5: Operation.
ISO 14644-6: Terminology.
ISO 14644-7: Separation devices.
ISO 14644-8: Molecular air pollution.
ISO 14644-9: Classification of surface cleaning.
ISO 14644-10: Chemical contamination (surfaces).
ISO 14644-12: Classification by concentration of nano particles.
ISO 14644-1
In accordance with the first section (of this standard, 9 categories are established, from ISO Class 1 to ISO Class 9, the first being the most restrictive
Characteristics and classification of clean rooms
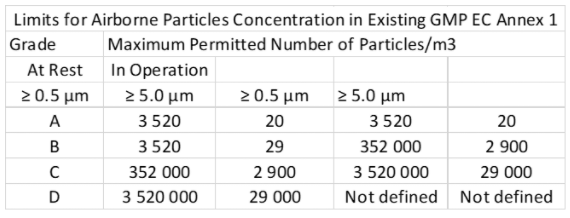
GMP refers to the Good Manufacturing Practice regulations promulgated by the US Food and Drug Administration. Subchapters A, B, C, D, and E for drugs and devices.)
The GMP (EU), Regulation 1223/2009 refers the requirements for clean rooms. This ensures that they are made in optimal conditions.
Like ISO STANDARDS, they vary depending on the industry in question. Thus for example in the food industry, the containment of particles and bacteria is essential to guarantee the quality of the products
GMP Standard
GMP refers to the Good Manufacturing Practice regulations promulgated by the US Food and Drug Administration. Subchapters A, B, C, D, and E for drugs and devices)
The GMP (EU) Regulation 1223/2009 refers the requirements for clean rooms. This ensures that they are made in optimal conditions.
Like ISO STANDARDS, they vary depending on the industry in question. Thus, for example, in the food industry, the containment of particles and bacteria is essential to guarantee the quality of the products.